
State of the art tissue engineering
Body-own tissue, no rejection
Grown at location where needed
Through an implantable Medical Device
True platform technology
Strong IP protection
Clinical success
Clinical primary and secondary objectives met
Safe – no safety issues
Effective – no deviations from performance claims
Consistent with results from four animal models
Commercial launch
First applications in cardiovascular
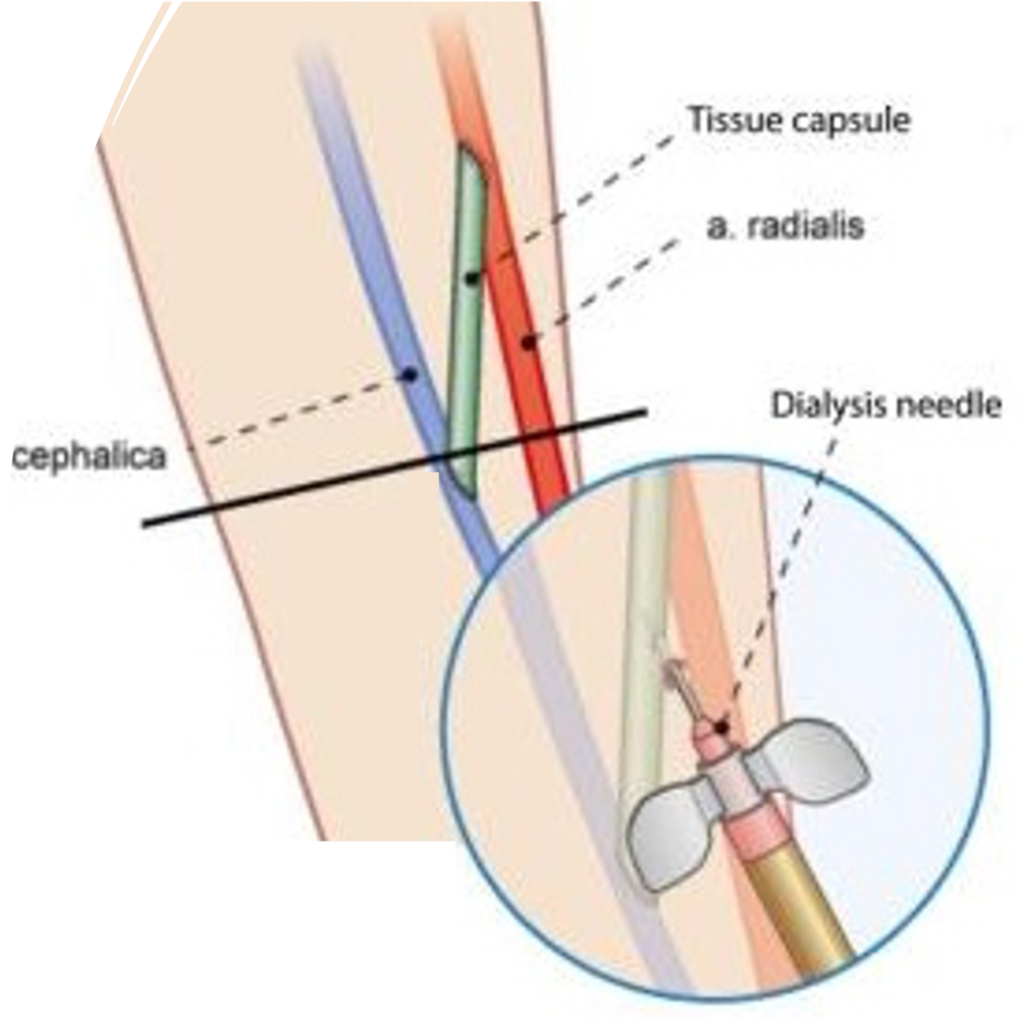
Initial focus on unmet need in hemodialysis

Quality Management System
CuroTis’ Quality Management System is audited and certified by Dekra
Certified by the European Union

