High level procedure
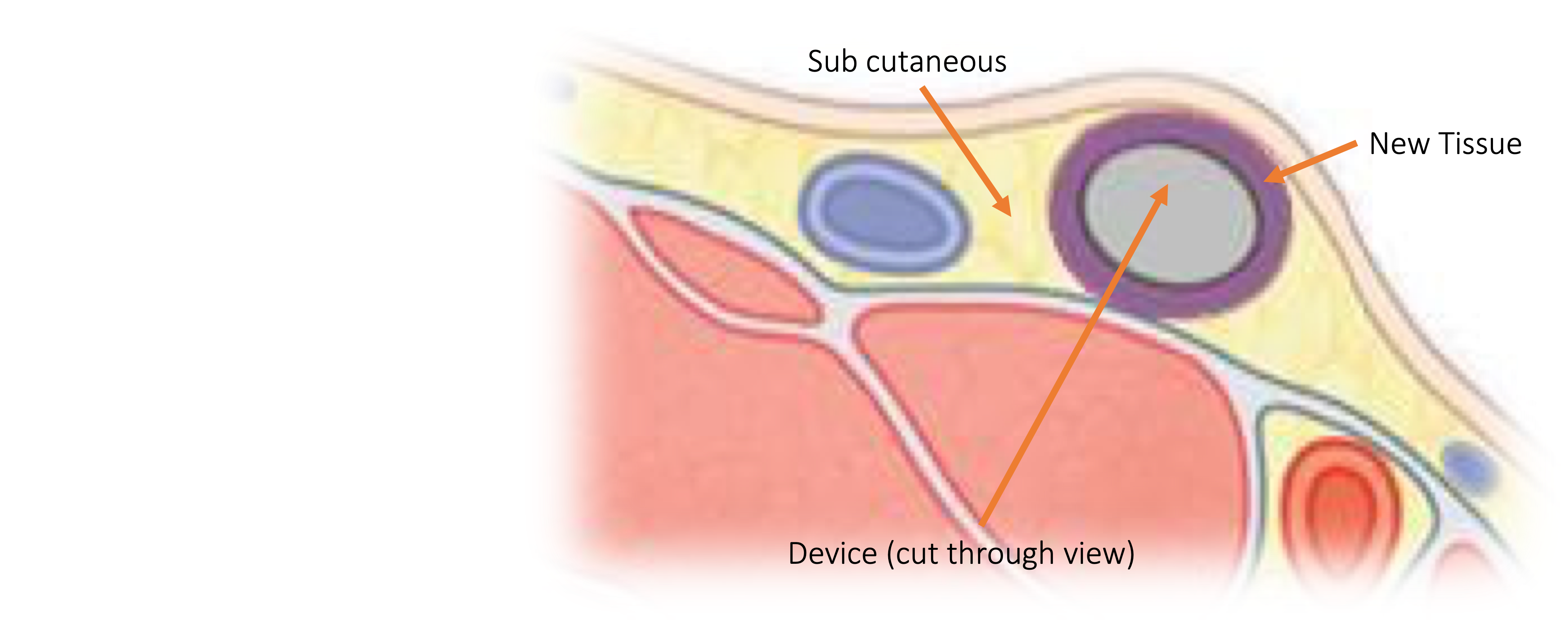
The CuroTis device is inserted under the skin
New tissue forms around it in the shape of the device
The tissue is created in the body at the anatomical location where it is needed
It is therefore body own and there is no rejection
The CuroTis device is easily removed after approximately one month

device regulated under the medical device regulation (MDR) in de EU

Dekra as Notified Body
Regulatory Pathway agreed
Class IIb (Rule 8)
Device regulated by fda in the usa

Regulatory Pathway agreed
Dedicated US team for FDA process
mechanism

The Curotis device comprises a biocompatible polymer with a defined surface structure
Upon implantation of the device the surface attracts selective proteins (a)
The proteins provide an anchor point for specific immune cells (b)
The immune cells respond with the secretion of specific cytokines and induce fibroblast to migrate into the emerging tissue (c)
Finally resulting in a layer of myofibroblasts that form a collagen matrix (d)
A new tissue is formed comprising a collagen extra cellular matrix incorporating myofibroblasts
publications
Tailoring the foreign body response for in situ vascular tissue engineering
Tissue Engineering 2014 link
Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix
Nature Scientific Reports 2014 link
Development and evaluation of in vivo tissue engineered blood vessels in a porcine model
Biomaterials 2016 link
Contribution of bone marrow-derived cells to in situ engineered tissue capsules in a rat model of chronic kidney disease
Biomaterials 2019 link
A novel method for engineering autologous non-thrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting
Biomaterials 2020 link
